by Sara Vallejo Castaño
Cement, the glue that holds our buildings together and the cornerstone of human settlements has a gas problem. As a rule of thumb, one ton of CO2 is emitted per ton of cement produced, contributing to 8% of global CO2 emissions [1]. In other words, if cement production was a country, it would be the third largest greenhouse gas (GHG) emitter in the world, right after China and the U.S [2].
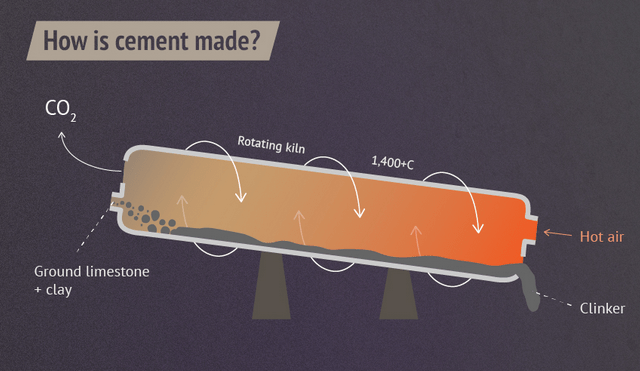
But decarbonizing cement production is not simple. Burning fossil fuels is necessary to transform minerals into cement at temperatures above 1300 °C (Figure 1). Additionally, even if these factories replace fossil fuels with hydrogen, the industry will still have a problem because 60% of cement’s GHG emissions come from the decomposition of limestone (CaCO3 → CaO + CO2), one of the major minerals used to produce cement.
So how do we transform the cement industry?
Professor Gaurav Sant, from the Civil and Environmental Engineering department at UCLA, has a solution. His scientific team has been working for over 5 years on a new cement formulation that is as strong as the industry standard (Portland cement) and that eludes the need for high temperature processing. One of the key ingredients in this novel formulation with cement-like binding properties is hydrated lime (Ca(OH)2). This material not only has been used in construction applications since Roman times, but it also has the capacity to absorb more than half of its weight in CO2 through the carbonation reaction: Ca(OH)2 + CO2 → CaCO3 + H2O. So instead of releasing CO2, this new formulation can be used as a sink to offset emissions from large industrial facilities such as thermal power plants (Figure 2).
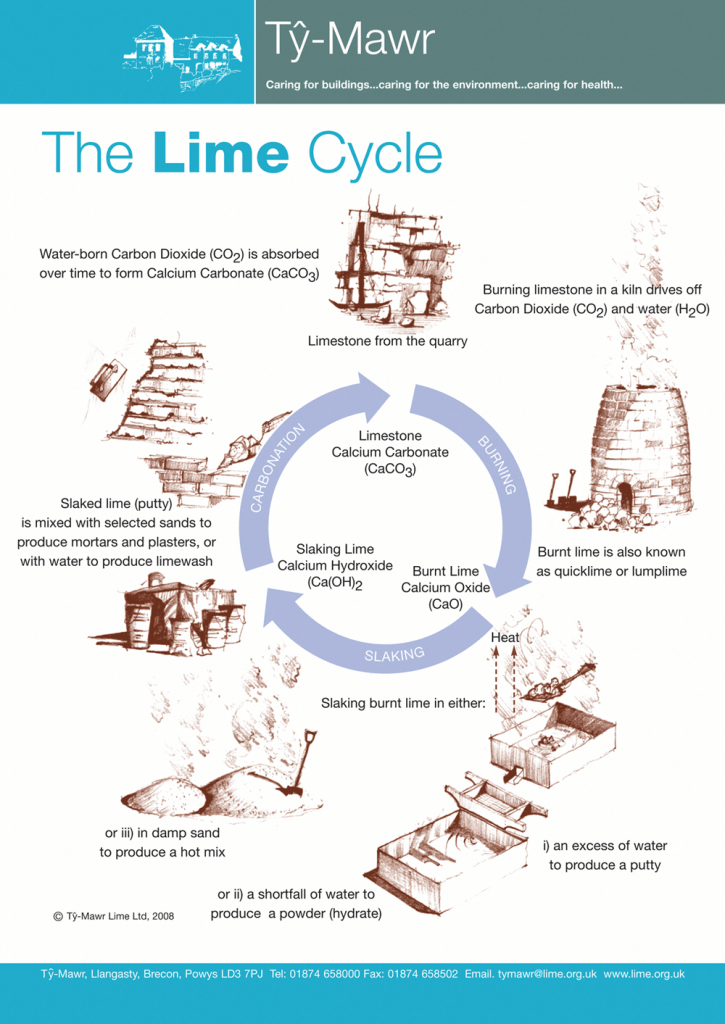
Figure 2. The lime cycle – How CaCO3 is converted to CaO and then to Ca(OH)2. (Source)
We still have a problem though. The majority of Ca(OH)2 is produced via limestone decomposition, the same troublesome reaction that produces CO2 in cement, which takes us back to square one (Figure 2). And this is where I come in. During the last 5 years, I have been working on a way to recycle calcium from the ashes of the iron and steel industry to produce hydrated lime. By recycling this underutilized by-product we can produce high quality hydrated lime (Ca(OH)2) at less than 100 °C using only electricity and waste heat. As a result, we end up with a “CO2-negative material”, a material that can capture more CO2 than what it produces during its lifecycle when renewable energy is the source of electricity (Figure 3). Incorporating this material into new cement formulations could be the key to radically transform the cement industry from being one of the main GHG emitters in the world to being a major carbon dioxide sink, just like a forest or an ocean.

Figure 3. Scanning electron microscopy image of Ca(OH)2 (large hexagon) synthesized from the ashes of steel . Additionally CaCO3 (small needles) impurities are observed from the carbonation of the Ca(OH)2 when in contact with air. [Original image]
Although this new material formulation has already been used to produce concrete blocks at industrial test sites, reclaiming calcium hydroxide from alkaline waste is still in the proof-of-concept stage. There is still a long road ahead. On the academic research front, avenues to improve calcium extraction and energy efficiency are still needed. In addition, for the commercial deployment of this technology, it will be crucial the support of industry and policy makers to develop regulation that incentivizes the production and consumption of this new generation of construction materials.
- J.M. Crow, The concrete conundrum, Chemistry World. (2008). https://www.chemistryworld.com/features/the-concrete-conundrum/3004823.article (accessed October 26, 2019).
- J. Timperley, Q&A: Why cement emissions matter for climate change, Carbon Brief. (2018). https://www.carbonbrief.org/qa-why-cement-emissions-matter-for-climate-change (accessed October 14, 2021).
Sara is part of the 2020-2021 INFEWS program cohort and a PhD candidate in Mechanical Engineering at UCLA. Her research focuses in developing an alternative method to produce hydrated lime using low-grade heat and alkaline byproducts from the steel industry.
The blog is part of the INFEWS Social Media Series and the Field Lab course.